
A molecule that is symmetrical and the atoms attached to the central atom have almost same electronegativity, then the molecule will be nonpolar.

It all depends on symmetry and electronegativity concept.

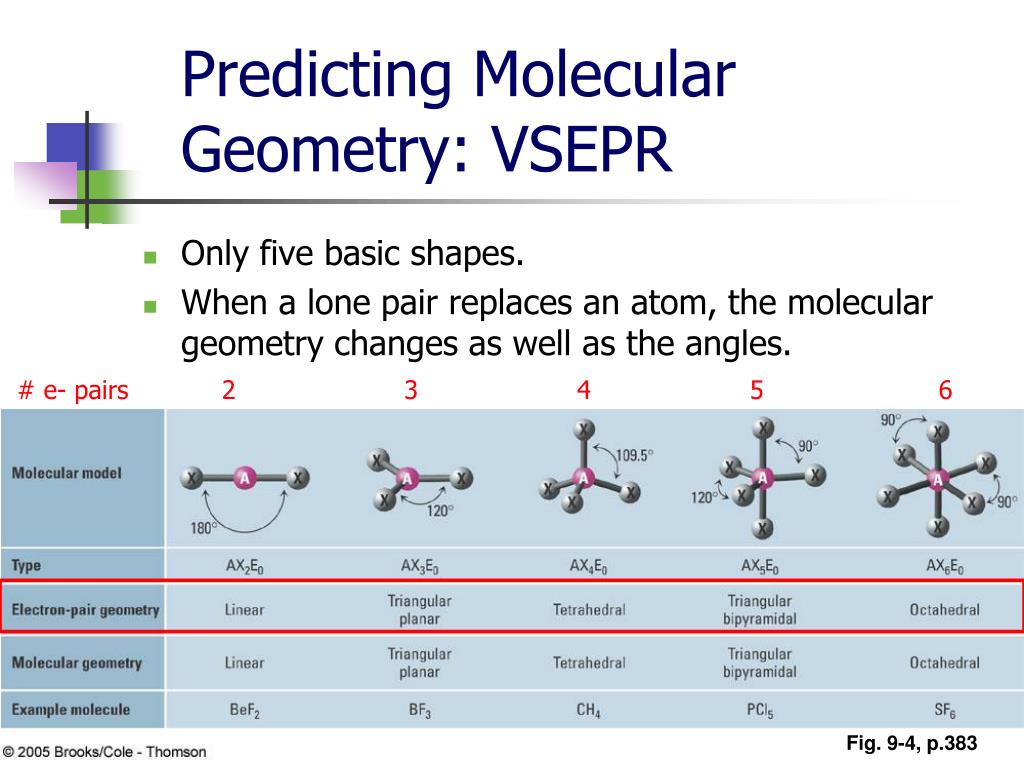
Shape:Ī tetrahedral molecule can be polar or nonpolar. The remaining four atoms connected to the central atom gives the molecule a square planar shape….Square Planar. The three factors governing polarity are shape, electronegativity and dipole moment. Tetrahedral is polar depending on geometry and can also be nonpolar. The shape of the molecule will determine the direction of each of the individual bond dipoles, and thus, will always play a role in determining the polarity of the molecule as a whole. How does molecular shape relate to polarity?ġ Answer. If the arrangement is asymmetrical, the molecule is polar.If the arrows are of different lengths, and if they do not balance each other, the molecule is polar.If the arrangement is symmetrical and the arrows are of equal length, the molecule is nonpolar.How do you determine the polarity of a shape? Lewis Structures and the Shapes of Molecules How do you determine the polarity of a shape?.PF 3Cl 2 - Phosphorus Trifluoride DichlorideĤ4. SF 5Cl - Sulfur Pentafluoride MonochlorideĤ1. SF 5Cl - Sulfur Monochloride PentafluorideĤ0. Step 3: Use symmetry to determine if the molecule is polar or non-polar.Ĭlick on the molecule's name to see the answer, but first try to do it yourself!ġ. Step 1: Draw the Lewis structure, Step 2: Draw the 3D molecular structure w/ VSEPR rules, Molecular Geometry & Polarity Example Problems VSEPR Rules: Electron and Molecular Geometry On Central Atom. VSEPR Rules: Table of Molecular Geometry, Molecular Polarity Problems (with 3D solutions!). And it's a good thing, because if water was not so polar, we would certainly not be here. Water, for example, is a very light molecule (lighter than oxygen gas or nitrogen gas) and you might expect it would be a gas based on its molecular weight, however the polarity of water makes the molecules "stick together" very well. when you compare it to other similar molecules. The polarity of a molecule will tell you a lot about its solubility, boiling point, etc. If the atoms are the same, the molecule is non-polar molecule if the atoms are different, the molecule is polar. Note: molecules with two atoms are not shown in these examples they are always linear with sp hybridization. If you click on the example molecules (where it says 3D view) below you'll get a better understanding of why some molecules are polar and some not. You need to consider the molecule in 3D (three dimensions).
Which vsepr shapes are polar how to#
To really understand how to do this, the Lewis structure is only the first step. However, if the molecule is asymmetric, the bond dipole moments won't "cancel out" and the molecule will have a net dipole moment and the molecule is therefore polar. Step 3: Determine if the molecular is polar or non-polar - a molecule is (i) non-polar if the charge distribution is symmetric and (ii) polar if the charge distribution is asymmetric (not symmetric).Īfter you draw the molecule in 3D representation using VSEPR rules, if the molecule has symmetry around the central atom, the bond dipole moments will "cancel out" (like pulling in opposite directions) and the molecule will therefore be non-polar. Note that double bonds and triple bonds count as a single region of electrons. Valence Shell Electron Pair Repulsion theory around the central atom all regions of electrons repel each other to get as far away from each other as possible while pivoting around the central atom. Note the number of electron regions around the central atom, and of these which are bonding or lone pairs (non-bonding pairs) Step 2: Use this info to determine the 3D geometry of the molecule. These are problems using 3D molecules run in the application Jmol to help you visualize the molecule to determine if it is polar or non-polar. How to Tell if a Molecule is Polar or Non-Polar VSEPR How To Tell if a Molecule Is Polar or Non-Polar?


 0 kommentar(er)
0 kommentar(er)
